Exoskeleton-Assisted Orthopedic Rehabilitation Technologies in 2025: Transforming Patient Outcomes and Shaping the Future of Mobility. Explore the Innovations, Market Dynamics, and Strategic Opportunities Driving This Rapidly Evolving Sector.
- Executive Summary: Key Trends and Market Drivers in 2025
- Market Size and Growth Forecast (2025–2030): CAGR and Revenue Projections
- Technological Innovations: Next-Gen Exoskeleton Designs and Capabilities
- Leading Companies and Industry Initiatives (e.g., eksoBionics.com, rewalk.com, suitx.com)
- Clinical Applications: Orthopedic Rehabilitation Use Cases and Patient Outcomes
- Regulatory Landscape and Standards (e.g., fda.gov, ieee.org)
- Integration with Digital Health and AI: Enhancing Therapy and Monitoring
- Regional Analysis: North America, Europe, Asia-Pacific, and Emerging Markets
- Investment, M&A, and Strategic Partnerships in Exoskeleton Rehab
- Future Outlook: Challenges, Opportunities, and the Road to Mainstream Adoption
- Sources & References
Executive Summary: Key Trends and Market Drivers in 2025
Exoskeleton-assisted orthopedic rehabilitation technologies are poised for significant growth and transformation in 2025, driven by advances in robotics, sensor integration, and artificial intelligence. These wearable robotic devices are increasingly being adopted in clinical and home settings to support patients recovering from musculoskeletal injuries, neurological disorders, and post-surgical rehabilitation. The global push for improved patient outcomes, reduced healthcare costs, and the growing prevalence of orthopedic conditions are key market drivers.
In 2025, the sector is witnessing a surge in both the sophistication and accessibility of exoskeleton systems. Leading manufacturers such as Ekso Bionics, ReWalk Robotics, and CYBERDYNE Inc. are expanding their product portfolios to address a broader range of rehabilitation needs, from lower limb support for stroke and spinal cord injury patients to upper limb exoskeletons for post-fracture therapy. These companies are also focusing on lightweight, modular designs and enhanced user interfaces, making devices more comfortable and easier to integrate into daily rehabilitation routines.
A notable trend in 2025 is the integration of real-time data analytics and cloud connectivity, enabling therapists to remotely monitor patient progress and adjust therapy protocols. For example, Ekso Bionics has incorporated advanced telemetry and feedback systems in its latest models, allowing for personalized rehabilitation programs and improved patient engagement. Similarly, ReWalk Robotics continues to refine its exoskeletons with enhanced gait training algorithms and remote support features.
Regulatory approvals and reimbursement pathways are also evolving, with several exoskeleton devices receiving clearances from health authorities in the US, Europe, and Asia. This regulatory momentum is expected to accelerate adoption in hospitals, rehabilitation centers, and even home care settings. Partnerships between device manufacturers and healthcare providers are facilitating large-scale clinical trials and real-world evidence generation, further validating the efficacy and safety of these technologies.
Looking ahead, the outlook for exoskeleton-assisted orthopedic rehabilitation technologies remains robust. The convergence of robotics, AI, and digital health is expected to yield even more intuitive, adaptive, and cost-effective solutions. As the global population ages and the incidence of orthopedic injuries rises, exoskeletons are set to become a cornerstone of modern rehabilitation, offering improved mobility, independence, and quality of life for millions of patients worldwide.
Market Size and Growth Forecast (2025–2030): CAGR and Revenue Projections
The global market for exoskeleton-assisted orthopedic rehabilitation technologies is poised for robust expansion between 2025 and 2030, driven by technological advancements, increasing prevalence of musculoskeletal disorders, and growing demand for advanced rehabilitation solutions. As of 2025, the sector is characterized by a dynamic landscape of established manufacturers and innovative startups, with North America, Europe, and parts of Asia-Pacific leading adoption.
Key industry players such as Ekso Bionics, ReWalk Robotics, CYBERDYNE Inc., and Hocoma AG are at the forefront, offering a range of exoskeletons designed for clinical and home-based orthopedic rehabilitation. These companies have reported increased demand from rehabilitation centers and hospitals, particularly for devices that assist in post-stroke, spinal cord injury, and lower limb orthopedic recovery.
Revenue projections for 2025 estimate the global exoskeleton-assisted rehabilitation market to reach approximately USD 1.2–1.5 billion, with a compound annual growth rate (CAGR) forecasted between 18% and 22% through 2030. This growth is underpinned by rising investments in healthcare infrastructure, favorable reimbursement policies in developed markets, and ongoing clinical validation of exoskeleton efficacy. For instance, Ekso Bionics and ReWalk Robotics have both announced expanded clinical partnerships and distribution agreements in 2024–2025, signaling confidence in sustained market growth.
The Asia-Pacific region is expected to witness the fastest CAGR, propelled by government initiatives to modernize rehabilitation services and a rapidly aging population. Meanwhile, Europe and North America will continue to dominate revenue share due to early adoption and established regulatory pathways. Notably, CYBERDYNE Inc. has expanded its presence in both Japan and Europe, leveraging its HAL (Hybrid Assistive Limb) technology for orthopedic and neurological rehabilitation.
Looking ahead, the market outlook remains optimistic as exoskeleton technologies become more affordable, lightweight, and user-friendly. Integration with digital health platforms and data analytics is anticipated to further enhance clinical outcomes and broaden adoption. By 2030, the sector is projected to surpass USD 3 billion in annual revenues, with continued innovation and cross-sector collaborations driving new applications in orthopedic rehabilitation.
Technological Innovations: Next-Gen Exoskeleton Designs and Capabilities
The field of exoskeleton-assisted orthopedic rehabilitation is experiencing rapid technological advancement as we enter 2025, with a focus on improving patient outcomes, device adaptability, and integration with digital health platforms. Next-generation exoskeletons are increasingly leveraging lightweight materials, advanced actuation systems, and artificial intelligence (AI) to deliver more personalized and effective rehabilitation experiences.
One of the most significant trends is the shift toward modular and customizable exoskeletons. Companies such as Ekso Bionics and ReWalk Robotics are developing devices that can be tailored to individual patient needs, allowing for targeted support of specific joints or limbs. These systems are designed to accommodate a wide range of orthopedic conditions, from post-stroke recovery to spinal cord injuries and post-operative rehabilitation.
Integration of AI and machine learning algorithms is another key innovation. These technologies enable real-time adaptation of exoskeleton assistance based on patient movement patterns and progress. For example, CYBERDYNE Inc. has incorporated bioelectrical signal detection in its HAL (Hybrid Assistive Limb) exoskeleton, allowing the device to interpret the user’s intention and adjust support accordingly. This approach enhances neuroplasticity and accelerates functional recovery.
Wireless connectivity and data analytics are also becoming standard features. Exoskeletons now often include sensors that collect biomechanical and physiological data, which can be transmitted to clinicians for remote monitoring and therapy adjustment. Hocoma, a subsidiary of DIH Medical, integrates cloud-based analytics with its Lokomat robotic gait training system, enabling evidence-based decision-making and personalized therapy plans.
In terms of hardware, the use of carbon fiber composites and miniaturized actuators is reducing device weight and improving user comfort. This is particularly important for outpatient and home-based rehabilitation, a segment expected to grow as healthcare systems emphasize decentralized care. Companies like SuitX (now part of Ottobock) are at the forefront of developing lightweight, ergonomic exoskeletons suitable for daily use outside clinical settings.
Looking ahead, the next few years are likely to see further convergence between exoskeletons and digital health ecosystems, including integration with tele-rehabilitation platforms and wearable health monitors. Regulatory approvals and reimbursement pathways are also evolving, with several devices already receiving clearances in major markets, paving the way for broader clinical adoption and accessibility.
Leading Companies and Industry Initiatives (e.g., eksoBionics.com, rewalk.com, suitx.com)
The exoskeleton-assisted orthopedic rehabilitation sector is experiencing rapid growth and innovation in 2025, driven by a combination of technological advancements, clinical validation, and expanding reimbursement pathways. Several leading companies are at the forefront, each contributing unique solutions and shaping the industry’s direction.
Ekso Bionics Holdings, Inc. remains a global leader in wearable exoskeleton technology for rehabilitation. Its flagship product, the EksoNR, is widely adopted in rehabilitation centers for patients recovering from stroke, spinal cord injury, and acquired brain injury. In 2024, Ekso Bionics announced expanded FDA clearances, enabling broader clinical use and supporting its integration into standard neurorehabilitation protocols. The company’s ongoing collaborations with major hospital networks and research institutions are expected to yield further clinical data and drive adoption through 2025.
ReWalk Robotics Ltd. continues to be a prominent player, particularly in personal exoskeletons for individuals with lower limb disabilities. The ReWalk Personal 6.0 system, designed for home and community use, has seen increased uptake following regulatory approvals in the U.S., Europe, and Asia. ReWalk Robotics is also advancing its ReStore soft exo-suit for post-stroke gait rehabilitation, with clinical studies underway to expand its indications and insurance coverage. The company’s strategic partnerships with rehabilitation clinics and insurers are expected to further accelerate market penetration in the coming years.
SuitX, now part of the Ottobock group, has leveraged its engineering expertise to develop modular exoskeletons for both medical and industrial applications. The SuitX Phoenix Medical Exoskeleton is designed for mobility-impaired users and is being evaluated in multiple clinical settings for its efficacy in improving gait and reducing secondary complications. Integration with Ottobock’s global distribution and support infrastructure is anticipated to enhance SuitX’s reach and accelerate product development cycles through 2025.
Other notable industry initiatives include CYBERDYNE Inc.’s HAL (Hybrid Assistive Limb) exoskeleton, which is gaining traction in Asia and Europe for both clinical and home rehabilitation, and Bionik Laboratories’ InMotion robotic systems, which are increasingly being integrated with exoskeleton platforms for comprehensive neurorehabilitation solutions.
Looking ahead, the sector is poised for further growth as clinical evidence mounts, device costs decrease, and digital health integration improves. Industry leaders are expected to focus on expanding indications, enhancing user experience, and forging partnerships with healthcare providers to ensure broader access and improved patient outcomes.
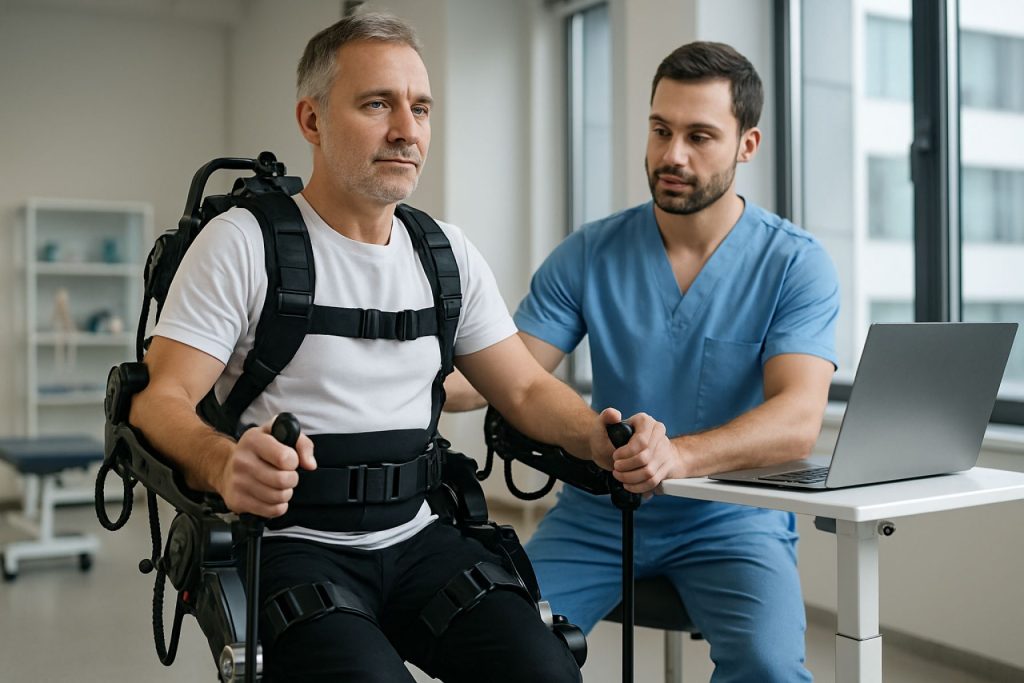
Clinical Applications: Orthopedic Rehabilitation Use Cases and Patient Outcomes
Exoskeleton-assisted orthopedic rehabilitation technologies are rapidly transforming clinical practice, offering new hope for patients recovering from musculoskeletal injuries, surgeries, and neurological impairments. As of 2025, these wearable robotic devices are increasingly integrated into rehabilitation protocols for conditions such as post-stroke hemiparesis, spinal cord injuries, hip and knee replacements, and traumatic orthopedic injuries. The primary clinical applications focus on restoring gait, improving lower and upper limb function, and accelerating the recovery timeline.
Leading manufacturers such as Ekso Bionics, ReWalk Robotics, and CYBERDYNE Inc. have developed FDA-cleared exoskeletons tailored for both clinical and personal use. For example, the EksoNR by Ekso Bionics is widely used in rehabilitation centers to assist patients with lower extremity weakness, enabling repetitive, task-specific gait training. Clinical studies and real-world deployments have shown that exoskeleton-assisted therapy can lead to significant improvements in walking speed, endurance, and independence compared to conventional therapy alone. In particular, patients with incomplete spinal cord injuries or post-stroke deficits have demonstrated enhanced neuroplasticity and functional gains when exoskeletons are incorporated early in the rehabilitation process.
Upper limb exoskeletons, such as those developed by Hocoma (a DIH company), are also gaining traction for shoulder, elbow, and wrist rehabilitation following orthopedic trauma or surgery. Devices like the ArmeoPower provide intensive, repetitive movement therapy, which is critical for regaining range of motion and strength. These systems often feature real-time feedback and data analytics, allowing therapists to tailor interventions and track patient progress with greater precision.
Recent years have seen a shift toward more compact, user-friendly, and cost-effective exoskeletons, making them accessible beyond specialized centers. Companies such as SuitX (now part of Ottobock), and Ottobock itself, are expanding their portfolios to include modular exoskeletons for both lower and upper limb support, targeting outpatient clinics and even home-based rehabilitation. This trend is expected to accelerate through 2025 and beyond, driven by advances in lightweight materials, battery technology, and AI-powered adaptive control systems.
Looking ahead, the outlook for exoskeleton-assisted orthopedic rehabilitation is highly promising. Ongoing clinical trials and multi-center studies are expected to provide robust data on long-term outcomes, cost-effectiveness, and patient satisfaction. As reimbursement pathways improve and device costs decrease, exoskeletons are poised to become a standard adjunct in orthopedic rehabilitation, offering improved mobility, reduced caregiver burden, and better quality of life for a growing patient population.
Regulatory Landscape and Standards (e.g., fda.gov, ieee.org)
The regulatory landscape for exoskeleton-assisted orthopedic rehabilitation technologies is rapidly evolving as these devices become more prevalent in clinical and home settings. In 2025, regulatory agencies and standards organizations are focusing on ensuring safety, efficacy, and interoperability, while also adapting to the unique challenges posed by wearable robotics.
In the United States, the U.S. Food and Drug Administration (FDA) continues to play a central role in the oversight of exoskeleton devices. Most lower-limb exoskeletons for rehabilitation are classified as Class II medical devices, requiring premarket notification (510(k)) to demonstrate substantial equivalence to a legally marketed predicate device. The FDA has cleared several exoskeletons for rehabilitation, including those from Ekso Bionics and ReWalk Robotics, setting important precedents for device classification and clinical evidence requirements. In 2024 and 2025, the FDA has signaled increased attention to post-market surveillance, cybersecurity, and human factors engineering, reflecting the growing complexity and connectivity of these systems.
Globally, the European Union’s Medical Device Regulation (MDR) continues to shape the approval process for exoskeletons, emphasizing clinical evaluation, risk management, and post-market vigilance. Companies such as CYBERDYNE Inc. and Hocoma AG have navigated these requirements to bring their devices to European markets. The MDR’s focus on real-world performance data and traceability is expected to influence regulatory approaches in other regions through 2025 and beyond.
Standards development is also advancing, with organizations like the IEEE and the International Organization for Standardization (ISO) working on frameworks for safety, performance, and interoperability. The IEEE has published standards such as IEEE 11073 for health informatics and is developing guidelines specific to wearable robotics, addressing issues like user interface, data security, and system reliability. ISO/TC 299 is similarly active in standardizing terminology and safety requirements for personal care robots, including exoskeletons.
Looking ahead, regulatory bodies are expected to further harmonize requirements, particularly regarding data privacy, remote monitoring, and integration with digital health records. The increasing use of artificial intelligence in exoskeleton control systems will likely prompt new guidance on algorithm transparency and validation. As the sector matures, collaboration between manufacturers, regulators, and standards organizations will be critical to ensuring that exoskeleton-assisted orthopedic rehabilitation technologies are both safe and accessible to patients worldwide.
Integration with Digital Health and AI: Enhancing Therapy and Monitoring
The integration of digital health platforms and artificial intelligence (AI) with exoskeleton-assisted orthopedic rehabilitation technologies is rapidly transforming the landscape of physical therapy and patient monitoring in 2025. Exoskeletons, once primarily mechanical aids, are now increasingly embedded with smart sensors, cloud connectivity, and AI-driven analytics, enabling more personalized, adaptive, and data-rich rehabilitation experiences.
Leading exoskeleton manufacturers are at the forefront of this convergence. Ekso Bionics has advanced its EksoNR platform with real-time data capture and remote monitoring capabilities, allowing clinicians to track patient progress, adjust therapy parameters, and intervene proactively. Their systems now integrate with digital health records, supporting seamless data flow between rehabilitation sessions and broader care teams. Similarly, ReWalk Robotics has enhanced its exoskeletons with wireless connectivity, enabling remote performance monitoring and tele-rehabilitation, which is particularly valuable for patients in remote or underserved areas.
AI algorithms are increasingly being used to analyze the vast datasets generated by exoskeleton sensors. These algorithms can detect subtle changes in gait, balance, and muscle activation, providing clinicians with actionable insights and early warnings of potential complications. For example, CYBERDYNE Inc. incorporates AI-driven feedback loops in its HAL (Hybrid Assistive Limb) exoskeletons, allowing the device to adapt in real time to the user’s neuromuscular signals and optimize assistance levels for each session.
Integration with digital health platforms is also enabling new models of care. Cloud-based dashboards and mobile applications allow patients and therapists to set goals, review progress, and communicate between sessions. This fosters greater patient engagement and adherence to rehabilitation protocols. Hocoma, a subsidiary of DIH Medical, has expanded its Lokomat and other robotic rehabilitation devices with digital health modules that support remote supervision and data-driven therapy adjustments.
Looking ahead, the next few years are expected to see further convergence of exoskeletons with AI-powered digital health ecosystems. Interoperability standards are being developed to ensure secure, privacy-compliant data exchange between devices and electronic health records. The use of predictive analytics is anticipated to enable even more proactive, personalized rehabilitation pathways, reducing recovery times and improving outcomes for orthopedic patients. As reimbursement models evolve to recognize the value of data-driven, remote-enabled care, adoption of these integrated exoskeleton solutions is poised to accelerate across hospitals, outpatient clinics, and home settings.
Regional Analysis: North America, Europe, Asia-Pacific, and Emerging Markets
The global landscape for exoskeleton-assisted orthopedic rehabilitation technologies is rapidly evolving, with distinct regional dynamics shaping adoption, innovation, and market growth. As of 2025, North America, Europe, and Asia-Pacific remain the primary hubs for technological advancement and clinical integration, while emerging markets are beginning to demonstrate increased interest and investment.
- North America: The United States and Canada continue to lead in both research and commercialization of exoskeleton-assisted rehabilitation. The region benefits from robust healthcare infrastructure, significant R&D funding, and a high prevalence of musculoskeletal disorders. Companies such as Ekso Bionics and ReWalk Robotics are at the forefront, with FDA-cleared devices for clinical and personal use. In 2024-2025, U.S. rehabilitation centers are increasingly integrating exoskeletons into post-stroke and spinal cord injury therapy, supported by growing insurance coverage and positive clinical outcomes. The Department of Veterans Affairs and leading hospitals are expanding pilot programs, further driving adoption.
- Europe: Europe is characterized by strong regulatory frameworks and collaborative research initiatives. Germany, Switzerland, and the Nordic countries are particularly active, with companies like Ottobock and Hocoma (a DIH company) developing advanced exoskeletons for both lower and upper limb rehabilitation. The European Union’s support for digital health and assistive technologies, along with reimbursement policies in countries such as Germany and France, is accelerating clinical adoption. In 2025, cross-border clinical trials and public-private partnerships are expected to further enhance technology validation and deployment.
- Asia-Pacific: The Asia-Pacific region is witnessing rapid growth, driven by aging populations and increasing healthcare investments. Japan and South Korea are notable for their early adoption, with companies like CYBERDYNE Inc. commercializing robotic exoskeletons such as HAL for rehabilitation in hospitals and eldercare facilities. China is also emerging as a significant player, with domestic manufacturers scaling up production and government initiatives supporting rehabilitation robotics. In 2025 and beyond, the region is expected to see expanded clinical trials and broader integration into public health systems.
- Emerging Markets: While adoption in Latin America, the Middle East, and Africa remains nascent, there is growing awareness of the benefits of exoskeleton-assisted rehabilitation. Pilot projects and partnerships with global manufacturers are underway, particularly in urban centers and private healthcare networks. As device costs decrease and local manufacturing capabilities improve, these regions are projected to experience gradual uptake over the next few years.
Overall, the outlook for exoskeleton-assisted orthopedic rehabilitation technologies is positive across all regions, with North America and Europe maintaining leadership, Asia-Pacific accelerating growth, and emerging markets poised for future expansion as accessibility improves.
Investment, M&A, and Strategic Partnerships in Exoskeleton Rehab
The exoskeleton-assisted orthopedic rehabilitation sector is experiencing a dynamic phase of investment, mergers and acquisitions (M&A), and strategic partnerships as of 2025. This activity is driven by the growing demand for advanced rehabilitation solutions, the maturation of core technologies, and the increasing integration of robotics into clinical practice. Key players are leveraging capital inflows and collaborations to accelerate product development, expand market reach, and enhance clinical efficacy.
In recent years, significant venture capital and institutional investment have flowed into leading exoskeleton developers. Ekso Bionics, a pioneer in wearable exoskeletons for rehabilitation, has continued to attract funding to support its R&D and global expansion. The company’s focus on hospital and outpatient rehabilitation centers has made it a preferred partner for healthcare providers seeking to modernize their therapy offerings. Similarly, ReWalk Robotics has secured additional investment to advance its exoskeleton platforms for both clinical and personal use, with a particular emphasis on post-stroke and spinal cord injury rehabilitation.
Strategic partnerships are a hallmark of the current landscape. Ottobock, a global leader in prosthetics and orthotics, has deepened its collaboration with exoskeleton innovators to integrate robotic assistance into its rehabilitation portfolio. The company’s acquisition of exoskeleton technology firms and its ongoing partnerships with hospitals and research institutions underscore its commitment to expanding the clinical utility of exoskeletons. Meanwhile, CYBERDYNE Inc. continues to form alliances with rehabilitation centers and academic institutions in Asia and Europe, facilitating clinical trials and the deployment of its HAL (Hybrid Assistive Limb) exoskeleton in diverse therapeutic settings.
M&A activity is also shaping the sector’s competitive dynamics. The acquisition of smaller, specialized robotics firms by established medical device companies is accelerating the integration of exoskeletons into broader rehabilitation ecosystems. For example, BIONIK Laboratories has pursued strategic acquisitions to enhance its product suite and strengthen its position in the North American and European markets.
Looking ahead to the next few years, the outlook for investment and strategic collaboration remains robust. The sector is expected to see continued inflows from both private and public sources, with a focus on scaling manufacturing, expanding clinical evidence, and achieving regulatory milestones. As reimbursement pathways become clearer and clinical adoption widens, exoskeleton-assisted orthopedic rehabilitation technologies are poised for accelerated growth, with partnerships and M&A serving as key enablers of innovation and market penetration.
Future Outlook: Challenges, Opportunities, and the Road to Mainstream Adoption
The future of exoskeleton-assisted orthopedic rehabilitation technologies in 2025 and the coming years is marked by both significant promise and notable challenges. As the global population ages and the incidence of musculoskeletal disorders rises, the demand for advanced rehabilitation solutions is expected to grow. Exoskeletons—wearable robotic devices designed to support or enhance limb movement—are increasingly being integrated into clinical practice, particularly for patients recovering from stroke, spinal cord injuries, or orthopedic surgeries.
Key industry players such as Ekso Bionics, ReWalk Robotics, and CYBERDYNE Inc. are at the forefront of developing medical exoskeletons tailored for rehabilitation. These companies have made significant strides in regulatory approvals and clinical deployments. For example, Ekso Bionics has received FDA clearance for its EksoNR exoskeleton for use in rehabilitation centers, while ReWalk Robotics continues to expand its presence in both clinical and personal use markets. CYBERDYNE Inc.’s HAL (Hybrid Assistive Limb) system is being adopted in hospitals across Asia and Europe, demonstrating the global reach of these technologies.
Despite these advances, several challenges remain on the road to mainstream adoption. High device costs, limited reimbursement pathways, and the need for specialized training for clinicians are persistent barriers. Additionally, the integration of exoskeletons into existing rehabilitation protocols requires robust clinical evidence demonstrating superior outcomes compared to conventional therapies. Ongoing studies and pilot programs in 2025 are expected to yield more data on long-term efficacy, patient adherence, and cost-effectiveness, which will be critical for broader acceptance by healthcare providers and payers.
Opportunities for growth are substantial. Technological advancements—such as improved battery life, lighter materials, and enhanced sensor integration—are making exoskeletons more user-friendly and adaptable to a wider range of patients. Companies like Hocoma (a division of DIH Medical) are innovating with modular and customizable systems that can be tailored to individual rehabilitation needs. Furthermore, collaborations between device manufacturers, hospitals, and research institutions are accelerating the development of evidence-based protocols and expanding access to these technologies.
Looking ahead, the convergence of artificial intelligence, tele-rehabilitation, and data analytics is poised to further enhance the capabilities of exoskeleton-assisted rehabilitation. As regulatory frameworks evolve and reimbursement models adapt, the sector is likely to see increased adoption in both inpatient and outpatient settings. By 2025 and beyond, exoskeletons are expected to become an integral component of orthopedic rehabilitation, offering improved mobility, faster recovery times, and better quality of life for patients worldwide.



