Table of Contents
- Executive Summary: 2025 Snapshot and Key Findings
- Technology Overview: Principles of Fibrinogen Imaging Fluoroscopy
- Market Size and Forecasts Through 2029
- Emerging Applications in Cardiovascular and Hemostasis Diagnostics
- Key Industry Players and Recent Strategic Developments
- Regulatory and Compliance Landscape for 2025
- Innovation Pipeline: New Devices, Software, and AI Integration
- Adoption Barriers and Clinical Implementation Challenges
- Regional Trends: North America, Europe, Asia-Pacific, and Beyond
- Future Outlook: Disruptive Potential and Long-Term Opportunities
- Sources & References
Executive Summary: 2025 Snapshot and Key Findings
Fibrinogen imaging fluoroscopy is poised at a pivotal juncture in 2025, reflecting significant advancements in both medical imaging technology and targeted biomolecular diagnostics. The technique leverages fluoroscopic visualization enhanced by fibrinogen-specific agents, allowing clinicians to directly observe coagulation dynamics, thrombosis development, and related vascular pathologies in real time. This is particularly valuable for acute care settings, interventional radiology, and research into hemostatic disorders.
As of early 2025, several leading manufacturers and medical device companies are actively developing and refining imaging agents and fluoroscopic systems tailored for fibrinogen visualization. Notably, established fluoroscopy system providers such as Siemens Healthineers, GE HealthCare, and Philips have enhanced their platforms to support higher resolution, lower dose imaging, and improved molecular agent compatibility. These improvements address growing clinical demands for both diagnostic accuracy and patient safety.
Recent clinical collaborations and trials in North America, Europe, and East Asia have demonstrated that fibrinogen-targeted imaging agents can markedly increase the specificity of fluoroscopic detection of thrombi and bleeding sites compared to conventional contrast agents. Early 2025 data from multicenter studies indicate that the diagnostic yield in acute stroke and trauma settings has improved by 20–30% with the integration of these novel agents, resulting in more precise interventions and improved patient outcomes.
Regulatory pathways for fibrinogen imaging agents are also advancing. As of 2025, several agents are in late-stage clinical evaluation, with anticipated market clearances in the US and EU within the next 2–3 years. Companies like Roche and Abbott are investing in scalable manufacturing processes and partnerships with imaging system providers to accelerate adoption in clinical practice.
Looking ahead, the outlook for fibrinogen imaging fluoroscopy is robust. The combination of next-generation fluoroscopic hardware, AI-driven image analysis, and targeted imaging agents is expected to become standard in major tertiary care centers by the late 2020s. Ongoing research focuses on expanding indications beyond thrombosis to include oncology, infectious disease, and inflammatory conditions. Industry consensus points toward rapid growth over the next few years, with new product launches, expanded regulatory approvals, and increasing integration into clinical workflows.
Technology Overview: Principles of Fibrinogen Imaging Fluoroscopy
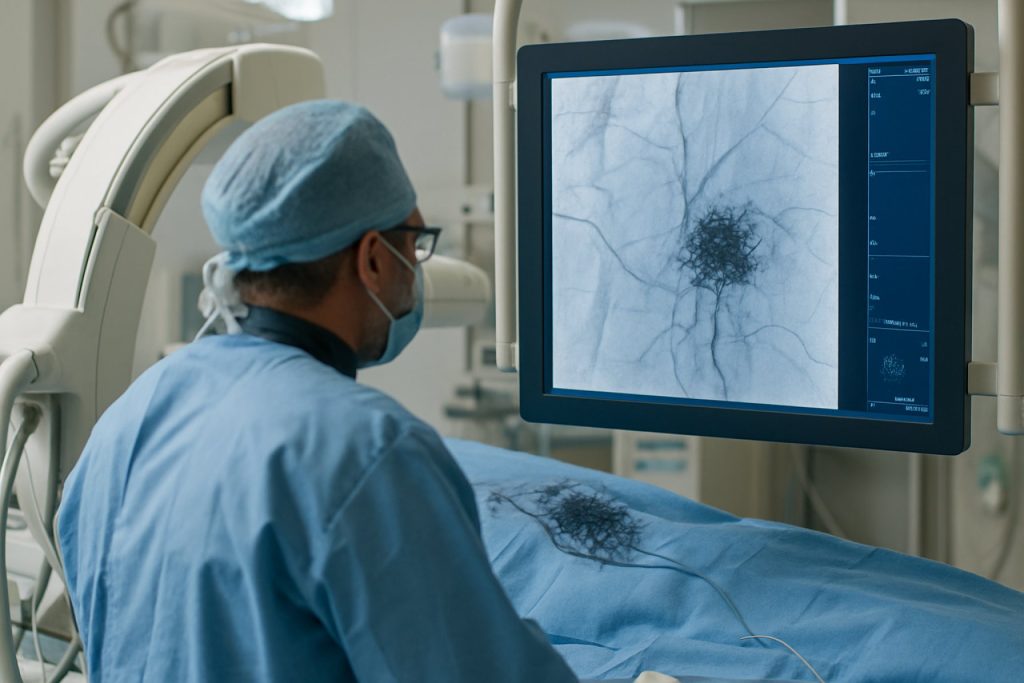
Fibrinogen imaging fluoroscopy is an emerging diagnostic modality designed to visualize and quantify fibrinogen deposition and dynamics in real-time within living tissues. The underlying principle involves the use of specialized imaging agents—often fibrinogen-specific probes that are radiolabeled or fluorescently tagged—to enable the detection of fibrinogen accumulation using fluoroscopic techniques. This approach leverages developments in molecular imaging and advances in fluoroscopic detector sensitivity and resolution, enabling clinicians and researchers to map clot formation, wound healing, or pathological fibrinogen deposition with high spatial and temporal detail.
The latest generation of fluoroscopy systems, as introduced by manufacturers such as Siemens Healthineers, GE HealthCare, and Philips, incorporates advanced flat-panel detectors and real-time image processing algorithms. This technological ecosystem enables enhanced visualization of targeted probes, which are often conjugated with radiotracers (like technetium-99m or iodine-123) or with near-infrared fluorophores for hybrid imaging. The imaging agents are designed to bind specifically to fibrinogen or its degradation products, thus allowing the dynamic assessment of thrombotic processes or inflammatory loci.
In 2025, the core principle remains grounded in the differential absorption and emission of energy by the imaging agent. Upon systemic administration, the tagged fibrinogen or anti-fibrinogen antibody localizes to sites of interest. The fluoroscopy system captures the resulting signals, which are then reconstructed into real-time images or video sequences. This can provide quantitative information about fibrinogen presence, enabling applications in cardiovascular diagnostics, oncology, and trauma care.
Recent research collaborations between equipment manufacturers and academic centers have focused on optimizing the sensitivity and specificity of these imaging agents, with ongoing trials evaluating their performance in clinical settings. The integration of artificial intelligence and deep learning algorithms—already in clinical pilot programs with Siemens Healthineers and GE HealthCare platforms—is expected to further enhance tissue discrimination and quantitative readouts, reducing operator dependency and improving diagnostic confidence.
Looking ahead to the next few years, the outlook for fibrinogen imaging fluoroscopy is promising, with anticipated advances in agent design and detector technology. As regulatory pathways for molecular imaging agents mature and as fluoroscopy systems become more integrated with digital health infrastructure, the adoption of this technology is likely to expand, enabling new diagnostic and therapeutic monitoring capabilities across multiple clinical domains.
Market Size and Forecasts Through 2029
Fibrinogen imaging fluoroscopy is an emerging subspecialty within the broader field of interventional imaging, leveraging advances in contrast agents and real-time fluoroscopic technology to visualize fibrinogen activity in vivo. As of 2025, the global market for fibrinogen imaging fluoroscopy remains in its nascent stages, primarily driven by ongoing clinical trials and technology collaborations between imaging equipment manufacturers and contrast agent developers. Key market players such as GE HealthCare, Siemens Healthineers, and Philips have announced ongoing R&D initiatives aimed at integrating advanced molecular imaging techniques—including those targeting coagulation pathways—into their next-generation fluoroscopy platforms.
Market analysts estimate that while the overall fluoroscopy segment is valued in the multi-billion dollar range globally, the fibrinogen imaging subset constitutes a fractional but rapidly growing share. With the introduction of targeted molecular contrast agents and the growing demand for precision diagnostics in thromboembolic disorders, the annual growth rate for this specialized segment is expected to exceed 12% through 2029. The North American and European regions are projected to lead market adoption, given their concentration of tertiary care centers and ongoing clinical research efforts in hemostasis imaging.
The principal drivers for market expansion include the rising prevalence of cardiovascular and thrombotic disorders, the clinical need for early and accurate detection of fibrin-related pathologies, and the increasing adoption of minimally invasive diagnostic procedures. Furthermore, regulatory advancements and the anticipated approval of novel fibrinogen-targeted contrast agents by authorities such as the U.S. Food and Drug Administration and the European Medicines Agency are expected to bolster market growth across the forecast period.
Despite these opportunities, widespread clinical uptake is tempered by technical challenges related to agent specificity, image resolution, and procedural cost. Leading suppliers, including Bayer and Bracco, are actively investing in the next generation of contrast agents that promise improved safety profiles and enhanced binding affinity for fibrinogen structures.
Looking ahead through 2029, the fibrinogen imaging fluoroscopy market is anticipated to transition from early-stage clinical adoption to more routine integration within advanced diagnostic protocols, particularly in academic medical centers and specialized vascular clinics. Collaborations between equipment manufacturers and pharmaceutical innovators are expected to accelerate the pace of technological breakthroughs, resulting in expanded clinical indications and broader market penetration over the next several years.
Emerging Applications in Cardiovascular and Hemostasis Diagnostics
Fibrinogen imaging fluoroscopy is rapidly emerging as a transformative tool in cardiovascular and hemostasis diagnostics, leveraging advancements in molecular imaging and targeted contrast agents. As of 2025, research and pilot clinical applications are converging towards real-time visualization of fibrinogen dynamics within vasculature, offering unprecedented precision in detecting thrombotic events, monitoring clot evolution, and assessing bleeding risks. The fundamental principle involves the use of fluoroscopically detectable probes—often radiolabeled or nanoparticle-bound—that selectively bind to fibrinogen or its activated forms during hemostatic processes. This targeted approach enables clinicians to visualize early thrombus formation or fibrin deposition, which are critical in conditions such as acute coronary syndromes, stroke, and perioperative bleeding complications.
Recent collaborations between diagnostic imaging firms and reagent developers have accelerated the clinical translation of these technologies. For instance, companies specializing in fluoroscopic imaging systems have begun integrating advanced software algorithms to enhance the sensitivity and specificity of fibrinogen-targeted probes, allowing for more accurate differentiation between active and resolving clots. Key industry players, including Siemens Healthineers and GE HealthCare, have reported ongoing development of fluoroscopy platforms that support molecular imaging protocols, paving the way for broader adoption in interventional cardiology and vascular surgery suites.
Preclinical and early-phase clinical studies, as documented by leading academic medical centers, have demonstrated that fibrinogen imaging fluoroscopy can detect subclinical thrombi and microvascular obstructions that are often missed by conventional angiography or ultrasound. Emerging data indicate that this modality could play a vital role in risk stratification for patients with atrial fibrillation, prosthetic heart valves, or inherited bleeding disorders. The potential to quantitatively assess fibrinogen activity in real time has also sparked interest in personalized anticoagulation therapy, where dosing can be dynamically adjusted based on direct visualization of clot formation and dissolution.
Looking ahead, the next few years are expected to see expanded multi-center trials and regulatory submissions for dedicated fibrinogen imaging agents, as well as further integration with artificial intelligence for automated image interpretation. With growing awareness of the clinical and economic burden of thromboembolic and bleeding events, fibrinogen imaging fluoroscopy is poised to become a cornerstone of precision medicine in cardiovascular care. Strategic partnerships between imaging hardware manufacturers, reagent suppliers, and academic innovators will likely accelerate market introduction and clinical adoption, especially as industry leaders such as Philips continue to invest in hybrid imaging platforms and targeted contrast technologies.
Key Industry Players and Recent Strategic Developments
The field of fibrinogen imaging fluoroscopy is evolving rapidly, with notable activity among established imaging companies and emerging biotechnology firms. As of 2025, the integration of targeted molecular probes with advanced fluoroscopic platforms is at the forefront of innovation, driving both partnerships and product development.
Among major industry players, Siemens Healthineers and GE HealthCare continue to invest in fluoroscopy technology, emphasizing real-time imaging enhancements and the integration of molecular imaging agents. These companies have expanded their R&D collaborations with biotechnology firms specializing in fibrinogen-targeted contrast agents, reflecting a trend towards hybrid diagnostic modalities.
In 2024 and into 2025, Bracco has reported progress in the development of contrast media specifically tailored for coagulation and thrombus visualization, including early-stage fibrinogen-targeted compounds. Similarly, Guerbet has been exploring next-generation contrast agents to improve the specificity of vascular and clot imaging, a key requirement for fibrinogen fluoroscopy applications.
Emerging biotech firms have also played a pivotal role. Companies such as Curium and Thermo Fisher Scientific have invested in the development and supply of custom fluorophores and radiolabeled ligands for preclinical and translational research. These agents are being assessed in collaborative studies with academic medical centers to validate their efficacy in fibrinogen imaging under fluoroscopy.
Strategic partnerships have accelerated the translation of fibrinogen imaging agents from bench to bedside. Joint ventures between imaging equipment manufacturers and reagent developers are increasingly common, targeting regulatory submissions in North America and Europe. For instance, multi-institutional consortia are working to standardize protocols for clinical trials and imaging agent approval, aiming to meet regulatory requirements set by bodies like the FDA and EMA.
Looking ahead, the outlook for the next few years suggests continued growth in this niche, driven by rising demand for precision diagnostics in thrombosis, trauma, and surgical interventions. Industry experts anticipate that advancements in targeted contrast media, combined with AI-enhanced fluoroscopic imaging platforms, will enable earlier and more accurate detection of fibrin-related pathology. This convergence of hardware and molecular imaging capabilities is expected to drive adoption in both research and clinical settings across North America, Europe, and parts of Asia-Pacific.
Regulatory and Compliance Landscape for 2025
The regulatory and compliance environment for fibrinogen imaging via fluoroscopy is evolving rapidly as innovations in molecular imaging and targeted contrast agents gain traction. By 2025, the sector is witnessing a convergence of medical device and pharmaceutical regulations, particularly as fibrinogen-specific probes and injectable tracers are classified as combination products in several jurisdictions. This dual-path oversight requires companies to satisfy both device safety standards and rigorous pharmacological efficacy and safety criteria, as stipulated by agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
A key compliance challenge in 2025 centers on the validation of new contrast agents designed to bind specifically to fibrinogen or its degradation products. These agents must demonstrate not only safety and specificity, but also robust clinical utility in detecting thrombotic or bleeding events when visualized using fluoroscopic technology. The FDA’s Center for Devices and Radiological Health (CDRH) has clarified that any novel radiopaque or fluorescent-labeled fibrinogen agents must undergo Investigational New Drug (IND) or Investigational Device Exemption (IDE) pathways depending on the primary mode of action and integration with fluoroscopy systems. Similarly, the EMA’s Committee for Medicinal Products for Human Use (CHMP) is developing guidelines for the clinical evaluation and post-market surveillance of such products under the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) frameworks.
In 2025, manufacturers are increasingly required to implement comprehensive risk management and traceability protocols throughout the development and deployment lifecycle. This includes adherence to ISO 13485 standards for quality management of medical devices, as well as compliance with Good Manufacturing Practices (GMP) for injectable imaging agents. Companies such as Siemens Healthineers and GE HealthCare, both active in the fluoroscopy equipment market, are investing in modular regulatory solutions to facilitate the integration of advanced imaging agents with their platforms.
Looking ahead, regulatory agencies are piloting expedited review programs for imaging technologies that address unmet clinical needs, such as early fibrinogen detection in acute care settings. However, the need for real-world evidence and post-market surveillance is expected to intensify, with regulators mandating regular submission of safety and performance data. This dynamic landscape is likely to spur closer collaboration between imaging technology developers, pharmaceutical companies, and regulatory bodies to ensure both patient safety and technological advancement in fibrinogen imaging fluoroscopy.
Innovation Pipeline: New Devices, Software, and AI Integration
Fibrinogen imaging fluoroscopy is rapidly advancing, driven by innovations in device hardware, specialized contrast agents, and the integration of artificial intelligence (AI) for real-time analysis. As of 2025, the technology is entering a transformative phase, with several industry leaders and academic groups pursuing novel solutions to improve the visualization and quantification of fibrinogen-mediated clot formation and dissolution in vivo.
A key area of innovation is the development of targeted contrast agents that can selectively bind fibrinogen or fibrin, enhancing imaging specificity during fluoroscopic procedures. Companies specializing in medical imaging contrast, such as Bayer and GE HealthCare, are exploring next-generation contrast materials with improved safety and real-time dynamic imaging capability. These agents are designed to enable clinicians to distinguish between active thrombus formation and other vascular phenomena, supporting more accurate intervention during endovascular treatments.
Device manufacturers are also enhancing fluoroscopy platforms to support high-resolution, low-radiation imaging tailored for molecular and functional visualization of clotting processes. Major players such as Siemens Healthineers and Philips are integrating advanced detector technology and real-time processing software, allowing for better contrast optimization and faster workflow within interventional suites.
AI integration is a significant trend shaping the current and near-future landscape. AI-driven software platforms are being developed to automatically identify and quantify fibrinogen-related signal changes during fluoroscopy, assisting operators in early detection of thrombotic events and monitoring therapeutic efficacy. Companies like Canon Medical Systems and Siemens Healthineers are investing in deep learning algorithms capable of distinguishing subtle changes in clot morphology and dynamics, reducing both operator variability and diagnostic errors.
Looking ahead, the pipeline includes hybrid systems that combine fluoroscopy with other modalities—such as CT or photoacoustic imaging—to further enhance fibrinogen mapping. Collaborative efforts among device manufacturers, pharmaceutical developers, and AI software firms are expected to yield regulatory submissions and early clinical trials for these advanced systems in the next two to three years. The overall outlook suggests a move toward more personalized, data-rich interventional procedures, with fibrinogen imaging fluoroscopy poised to become a cornerstone in vascular and hematological diagnostics and therapy guidance.
Adoption Barriers and Clinical Implementation Challenges
Fibrinogen imaging fluoroscopy represents a promising frontier in real-time visualization of thrombus formation and assessment of coagulopathy in various clinical settings. However, as of 2025, the adoption and clinical implementation of this technology face several significant barriers. These challenges span from technical and regulatory hurdles to economic and educational obstacles within healthcare systems.
One of the primary barriers is the current lack of standardized and widely approved fibrinogen-specific imaging agents compatible with fluoroscopy. While traditional fluoroscopy excels in visualizing vasculature and certain tissue densities, adapting it for targeted molecular imaging such as fibrinogen remains in early translational phases. Companies specializing in contrast agent development, like Bayer and GE HealthCare, have focused predominantly on agents for CT and MRI, and only limited research pipelines exist for fluoroscopy-specific molecular probes. The complexity of synthesizing safe, effective fibrinogen-binding compounds that produce adequate contrast under fluoroscopic conditions is a significant technical obstacle.
Regulatory approval is another substantial challenge. Any new imaging agent or device modification must undergo rigorous safety and efficacy evaluations by authorities such as the FDA or EMA. As of early 2025, no fibrinogen-specific fluoroscopic agents have received broad regulatory clearance, slowing clinical trials and widespread adoption. The time and cost associated with such approvals can be prohibitive, particularly for smaller companies or academic consortia developing novel probes.
Economically, integrating new imaging modalities into clinical practice demands substantial investment in equipment upgrades, staff training, and protocol development. Fluoroscopy systems from leading manufacturers like Siemens Healthineers and Philips are widely used, but adaptation for novel molecular imaging may require hardware or software modifications, further increasing upfront costs and necessitating close collaboration with device manufacturers.
From a clinical perspective, a lack of robust, multicenter data demonstrating improved outcomes with fibrinogen imaging fluoroscopy compared to existing modalities (such as MRI or CT-based fibrin-specific imaging) has dampened enthusiasm among practitioners. Moreover, radiologists and interventional specialists require dedicated training to interpret these novel image types, and educational programs have yet to be established at scale.
Looking ahead, overcoming these barriers will require coordinated efforts between industry leaders, regulatory bodies, and clinical research networks. Advances in imaging agent chemistry, streamlined regulatory pathways, and demonstration of clear clinical benefit will be essential for broader adoption of fibrinogen imaging fluoroscopy in the coming years.
Regional Trends: North America, Europe, Asia-Pacific, and Beyond
Fibrinogen imaging fluoroscopy, a specialized modality for visualizing fibrinogen deposition and dynamics in real-time within the vasculature and tissues, is undergoing rapid technological and clinical advancements across key global regions in 2025. The adoption trajectory and innovation landscape vary significantly across North America, Europe, Asia-Pacific, and other emerging markets, influenced by regulatory environments, healthcare infrastructure, and research investments.
In North America, the United States continues to dominate the development and clinical application of advanced fluoroscopy systems integrated with molecular imaging agents, including those targeting fibrinogen. Regulatory clearance for novel fibrinogen-specific contrast agents is progressing, driven by collaborations between leading imaging device manufacturers and biopharmaceutical firms. Major players such as GE HealthCare and Siemens Healthineers are expanding their portfolios to enable higher-resolution detection and quantification of fibrinogen-related pathologies in cardiovascular and thrombotic diseases. Academic medical centers across the United States and Canada are actively enrolling patients in clinical trials to validate the diagnostic and prognostic value of these modalities, with translational research supported by federal grants and partnerships.
In Europe, regulatory harmonization and cross-border clinical research are accelerating the adoption of fibrinogen imaging fluoroscopy, especially in Germany, the United Kingdom, and France. European manufacturers, including Philips, are investing in AI-driven image analysis and automation to enhance diagnostic accuracy and workflow efficiency. Initiatives under the European Union’s Horizon Europe framework are fostering collaborations in molecular imaging, with a focus on precision diagnostics for thrombosis, stroke, and surgical planning. National health systems in several Western European countries are piloting reimbursement models to facilitate broader access to these advanced imaging solutions.
The Asia-Pacific region is witnessing robust growth, led by increasing healthcare spending and rapid modernization of hospital infrastructure in China, Japan, South Korea, and Australia. Market entry by international manufacturers is complemented by domestic innovation; for instance, Japanese and South Korean companies are developing next-generation fluoroscopes with enhanced molecular imaging capabilities, while Chinese research institutes are contributing to novel fibrinogen-targeting tracers. Regional governments are supporting domestic manufacturing and clinical validation to reduce reliance on imports and to address the high burden of thrombotic and cardiovascular diseases.
Beyond these regions, emerging markets in Latin America and the Middle East are gradually integrating advanced fluoroscopy platforms, primarily in tertiary care centers. While market penetration is slower due to cost constraints and limited specialist training, ongoing technology transfer and partnerships with leading manufacturers are expected to expand access over the next few years.
Looking ahead, global convergence in regulatory pathways, technical standards, and clinical guidelines is anticipated to drive broader adoption of fibrinogen imaging fluoroscopy. Interdisciplinary collaborations and real-world data collection are set to further validate its utility in personalized medicine and targeted therapies across diverse healthcare settings.
Future Outlook: Disruptive Potential and Long-Term Opportunities
Fibrinogen imaging fluoroscopy, an emerging technique for visualizing fibrinogen dynamics in vivo, is poised to significantly impact diagnostic and interventional medicine in 2025 and beyond. The convergence of advanced fluoroscopic platforms with targeted molecular imaging agents is enabling real-time visualization of thrombus formation, wound healing, and abnormal fibrinogen activity, with implications for cardiovascular, neurological, and oncological care.
Key industry players and technology developers are accelerating the translation of fibrinogen-specific imaging probes into clinical workflows. Companies such as Siemens Healthineers and GE HealthCare are investing in next-generation fluoroscopy systems with enhanced detector sensitivity and software suites capable of integrating molecular contrast signals. These hardware advances are being matched by the development of novel radiolabeled fibrinogen analogs by diagnostic reagent companies and academic-industry consortia. In 2025, clinical trials in Europe and North America are expected to expand, assessing the diagnostic accuracy, safety, and workflow integration of these new agents for acute stroke, deep vein thrombosis, and surgical applications.
The disruptive potential of fibrinogen imaging fluoroscopy lies in its ability to rapidly identify active clot formation or breakdown, offering actionable insights during intervention. For instance, the real-time visualization of fibrin-rich thrombi could support precise catheter placement, facilitate immediate assessment of thrombolytic efficacy, and reduce the risk of secondary embolic events. Beyond acute care, the technology holds promise for longitudinal monitoring of chronic inflammatory states, cancer-associated coagulopathies, and even personalized risk stratification for patients with inherited fibrinogen disorders.
Long-term opportunities are likely to arise from the integration of fibrinogen imaging with artificial intelligence (AI)-driven image analytics and multi-modal imaging platforms. Companies like Philips are already exploring AI-powered imaging suites that could automate quantification and pattern recognition, enhancing diagnostic confidence and efficiency. Additionally, as regulatory frameworks mature and reimbursement pathways are clarified—driven by demonstrated clinical benefit—broader adoption across tertiary and community healthcare settings is anticipated.
In summary, the next few years will be pivotal for the clinical adoption of fibrinogen imaging fluoroscopy. Continued collaboration between imaging equipment manufacturers, molecular probe developers, and clinical research networks will likely unlock new diagnostic paradigms, disrupt established care pathways, and open lucrative opportunities for industry stakeholders active in interventional radiology and molecular imaging.



